세미나
Physicochemical Analysis of Infiltrated Cathode Symmetric Cells for Intermediate Temperature SOFCs
- 일시 2022-09-23 16:00 ~ 18:00
- 장소 충무관 106호
- 연사 송선주 교수님
- 소속 전남대학교
-
첨부파일이 없습니다.
Lowering the operating temperature of the solid oxide fuel cell is important to prevent material degradation but causes a high polarization problem that slows down the electrode kinetics. The infiltration of multi-cation oxide nanoparticle catalysts on the cathode backbone can provide low activation energies to catalyze several electrochemical processes. In this study, Sm0.5Sr0.5CoO3-δ(SSC55) nanoparticles were infiltrated on the surface of the La0.6Sr0.4Co0.2Fe0.8O3-δ(LSCF6428) backbone pasted on the GDC electrolyte. Electrochemical impedance (EI) spectra of the cells before and after infiltration were measured under the various thermodynamic conditions (700~600℃, pO2=0.21~0.02atm). The parameters (Rs,Rp,Cchem,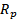 and CHN0
and CHN0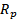 ) of the transmission line model (TLM) expressing the polarization of the cathode were analyzed in detail. Rs
) of the transmission line model (TLM) expressing the polarization of the cathode were analyzed in detail. Rs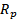 is the diffusion resistance of the bulk or surface and Rp
is the diffusion resistance of the bulk or surface and Rp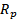 is the resistance of surface reactions such as the decomposition resistance of gas molecules. Cchem
is the resistance of surface reactions such as the decomposition resistance of gas molecules. Cchem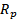 is the chemical capacitance resulting from the nonstoichiometry of the electrode material, and CHN0
is the chemical capacitance resulting from the nonstoichiometry of the electrode material, and CHN0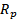 is the capacitance effect of the surface which varies with frequency. Trends of parameters according to the thermodynamic variables (temperature, pO2 and current density) were sufficiently discussed by analyzing the cells before and after infiltration, and how the impedance analysis should be performed to microstructure-modified electrodes was discussed.
is the capacitance effect of the surface which varies with frequency. Trends of parameters according to the thermodynamic variables (temperature, pO2 and current density) were sufficiently discussed by analyzing the cells before and after infiltration, and how the impedance analysis should be performed to microstructure-modified electrodes was discussed.



